Rotterdam, The Netherlands, June 21, 2021 (GLOBE NEWSWIRE) —
Summary:
- Centrient Pharmaceuticals has started production at its newly built statins API manufacturing unit in Toansa, India.
- With this expansion the company is doubling its production capacity of Atorvastatin and Rosuvastatin, meeting the increased demand for its high-quality uniquely produced statins.
- Centrient Pharmaceuticals’ statins are one of the most sustainably produced in the industry by eliminating harmful solvents, generating less waste, and a reduced carbon footprint of 32% as compared to traditional manufacturers.
- Using backward integrated manufacturing methods, and dedicated production facilities, Centrient Pharmaceuticals is able to offer its customers security of supply.
Centrient Pharmaceuticals (“Centrient”), the global leader in sustainable antibiotics, next-generation statins and anti-fungals, announced today to have started production at its new statins manufacturing unit. With the building of its second dedicated unit on the Toansa site in India now completed, the company will double its statins production capacity. This will enable Centrient to meet growing demand for its sustainably manufactured Atorvastatin and Rosuvastatin Active Pharmaceutical Ingredients (APIs).
Statins are currently the most prescribed drug class globally for the treatment of high cholesterol and cardiovascular diseases and are among the top-selling drugs worldwide. The markets for Atorvastatin and Rosuvastatin in particular, has shown steady growth in the past years, as a result of the continued global prevalence of high cholesterol issues, replacement of older generation statins, and genericization of the market.
Starting almost a decade ago, Centrient has grown today into one of the leading statin API suppliers worldwide, servicing large pharma companies around the globe.
Next to high-quality features like long shelf life and large batch sizes, the company offers security of supply to customers through its dedicated statins production facility and backward integration. Being backward integrated, Centrient is independent from external imports of starting materials. Its enzymatic route of synthesis and patented technology minimise the use of harmful solvents, generate less waste, and reduce the company’s carbon footprint by 32% as compared to traditional manufacturers.
The news of the facility expansion follows major milestones on statins that the company reached in the past years. In 2012, under the name of DSM Sinochem Pharmaceuticals, it was the first pharmaceutical manufacturer worldwide to offer generic Atorvastatin APIs under a Certificate of Suitability to the Monograph of the European Pharmacopoeia (CEP). Since 2014, it has produced the unique Atorvastatin APIs in its state-of-the-art facility in Toansa, India for third-party customers.
In addition, the company was one of the first three companies worldwide that started to offer generic Rosuvastatin APIs under CEP in 2016. Two years later, the first generic Rosuvastatin and Atorvastatin finished dosage forms were launched in Western Europe.
“With the doubling of our production capacity, we demonstrate our commitment to maintain our leadership position in line with our strategy and to continue supporting our customers’ business growth. Guided by our brand promise of Quality, Reliability, and Sustainability, Centrient’s Rosuvastatin and Atorvastatin offer superior performance in all three areas to the benefit of our customers and the environment.”, says Frans Vlaar, Chief Commercial Officer at Centrient.
Ground breaking of the new manufacturing unit started at the end of 2019 and commercial supplies from the new unit will start in mid-2021. With the new manufacturing line being operational and doubling the production capacity, Centrient will be even better positioned to secure supply, meeting the growing demand from customers and helping to improve the lives of patients who are in need of these medicine.
“We are extremely proud that we have been able to complete this project in a timely way given the challenges of executing such a complex project in the midst of the COVID pandemic,” says Jim McPherson, Chief Quality & Technical Operations Officer. “It reinforces our absolute commitment to meet the expectations of our customers as a partner of choice – delivering reliable and secure supply using leading sustainable technologies. The facility incorporates design features that allow further improvements in GMP and energy utilization, and enable greater automation for improved process control.”
——————————
About Centrient Pharmaceuticals
Centrient Pharmaceuticals is the leading manufacturer of beta-lactam antibiotics, and a provider of next generation statins and antifungals. We produce and sell intermediates, active pharmaceutical ingredients and finished dosage forms.
We stand proudly at the centre of modern healthcare, as a maker of essential and life-saving medicines. With our commitment to Quality, Reliability and Sustainability at the heart of everything we do, our over 2200 employees work continuously to meet our customers’ needs. We work towards a sustainable future by actively participating in the fight against antimicrobial resistance.
Founded 150 years ago as the ‘Nederlandsche Gist- en Spiritusfabriek’, our company was known as Gist Brocades and more recently DSM Sinochem Pharmaceuticals. Headquartered in Rotterdam (Netherlands), we have production facilities and sales offices in China, India, the Netherlands, Spain, Egypt, the United States and Mexico. Centrient Pharmaceuticals is wholly owned by Bain Capital Private Equity, a leading global private investment firm.
For more information please visit www.centrient.com or contact Centrient Pharmaceuticals Corporate Communications, Alice Beijersbergen, Director Branding & Communications. E-Mail: alice.beijersbergen@centrient.
Forward-looking statements
This press release may contain forward-looking statements with respect to Centrient Pharmaceuticals’ future financial performance and position. Such statements are based on current expectations, estimates and projections of Centrient and information currently available to the company. Centrient cautions readers that such statements involve certain risks and uncertainties that are difficult to predict and therefore it should be understood that many factors can cause actual performance and position to differ materially from these statements. Centrient has no obligation to update the statements contained in this press release, unless required by law. The English language version of the press release is governing.
Alice Beijersbergen Centrient Pharmaceuticals alice.beijersbergen@centrient.com


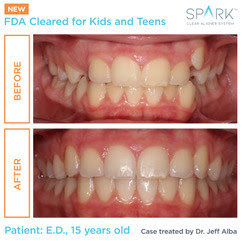
 “My son and I have been so happy with his experience using Spark Aligners,” said Mrs. Strecker, a parent of a 9-year-old who needed treatment for spacing as well as AP correction. “Our orthodontist recommended Spark because he achieved outstanding results with other patients and told us the material used in Spark Aligners would be the clearest option while also being more comfortable than the leading competitive alternative. Because people could barely notice he was wearing aligners, my son felt confident during his entire treatment. We saw his smile change dramatically in about 3 months and treatment completion with a beautiful smile in 5 months. We are big Spark fans!”
“My son and I have been so happy with his experience using Spark Aligners,” said Mrs. Strecker, a parent of a 9-year-old who needed treatment for spacing as well as AP correction. “Our orthodontist recommended Spark because he achieved outstanding results with other patients and told us the material used in Spark Aligners would be the clearest option while also being more comfortable than the leading competitive alternative. Because people could barely notice he was wearing aligners, my son felt confident during his entire treatment. We saw his smile change dramatically in about 3 months and treatment completion with a beautiful smile in 5 months. We are big Spark fans!”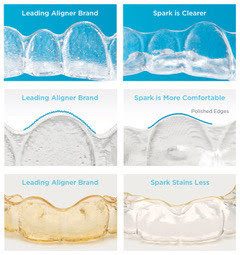 “My practice has been impressed with the results we are achieving for our patients with Spark Aligners,” said Dr. Bill Dischinger* of Dischinger Team Orthodontics. “The TruGEN material from which the aligners are made delivers better surface contact providing faster and more reliable tooth movements. With Spark Aligners, we’re seeing stronger and more predictable outcomes than we’ve achieved with other aligners, so the expanded indication of usage is great news for my younger patients who lead busy lifestyles filled with sports, music and other activities. My entire team and our patients are thrilled with the efficient, reliable treatment experience that Spark Aligners deliver.”
“My practice has been impressed with the results we are achieving for our patients with Spark Aligners,” said Dr. Bill Dischinger* of Dischinger Team Orthodontics. “The TruGEN material from which the aligners are made delivers better surface contact providing faster and more reliable tooth movements. With Spark Aligners, we’re seeing stronger and more predictable outcomes than we’ve achieved with other aligners, so the expanded indication of usage is great news for my younger patients who lead busy lifestyles filled with sports, music and other activities. My entire team and our patients are thrilled with the efficient, reliable treatment experience that Spark Aligners deliver.” “Spark Aligners were made in collaboration with leading orthodontists. This along with TruGEN™, the most advanced material available, ensures a better treatment experience for patients,” said Sheila Tan, Vice President of Global Marketing & Clinical Education. “We are delighted with the FDA clearance to expand our treatment options to include kids and teens. Now Spark Aligners can be used to treat young children that do not have all of their permanent teeth.”
“Spark Aligners were made in collaboration with leading orthodontists. This along with TruGEN™, the most advanced material available, ensures a better treatment experience for patients,” said Sheila Tan, Vice President of Global Marketing & Clinical Education. “We are delighted with the FDA clearance to expand our treatment options to include kids and teens. Now Spark Aligners can be used to treat young children that do not have all of their permanent teeth.”